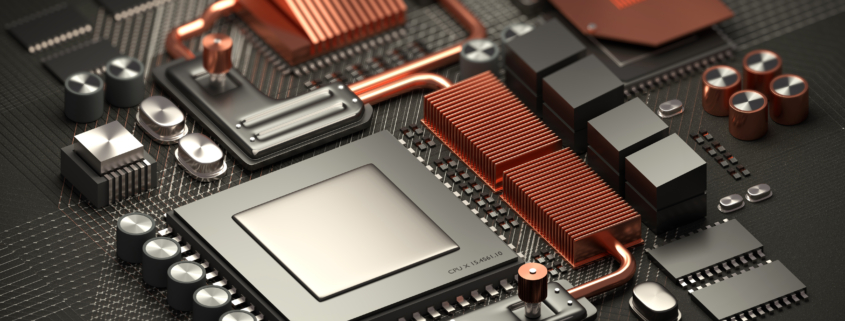
A typical sealed copper heat transfers the heat with minimal temperature difference and spreads the heat across the heat sinks or fins.
The evaporated liquid travels along the heat pipe from the heating point to the cold interface and condenses into a liquid.
The liquid returns to the heating point through either capillary action or gravity, and the cycle repeats.


